
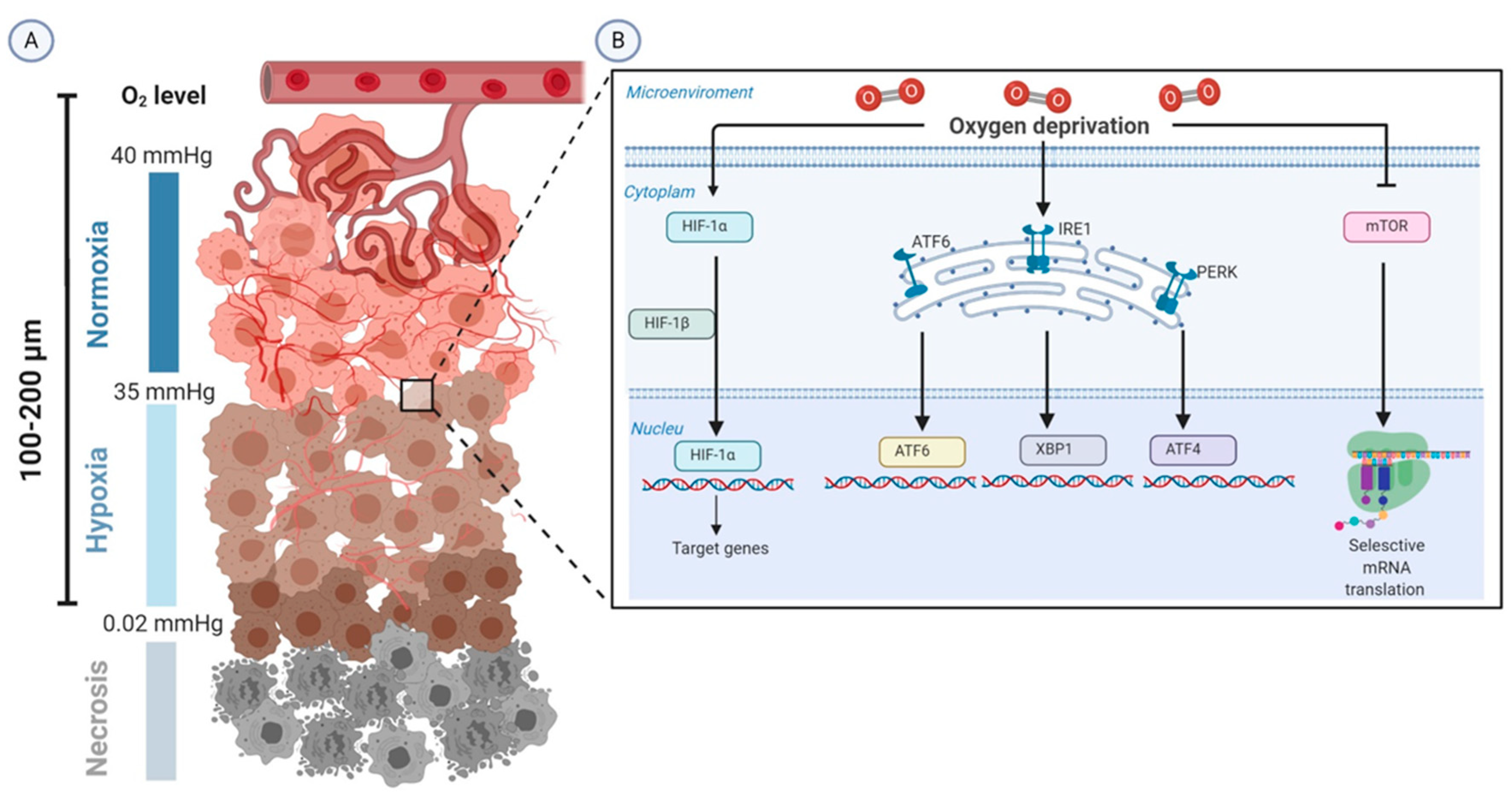
A 721 mL vessel contains Ne at a temperature of 36.2 ° C and a pressure of 0.185 atm. Express this pressure in atmospheres, kilopascals, torrs, pounds per square inch, and pascals. 31) A 1.37 L vessel contains He at a temperature of 24.5 ° C and a pressure of 205 mmHg. You can view more details on each measurement unit: atm or in Hg The SI derived unit for pressure is the pascal. We assume you are converting between atmosphere standard and.
#721 mmhg to atm full#
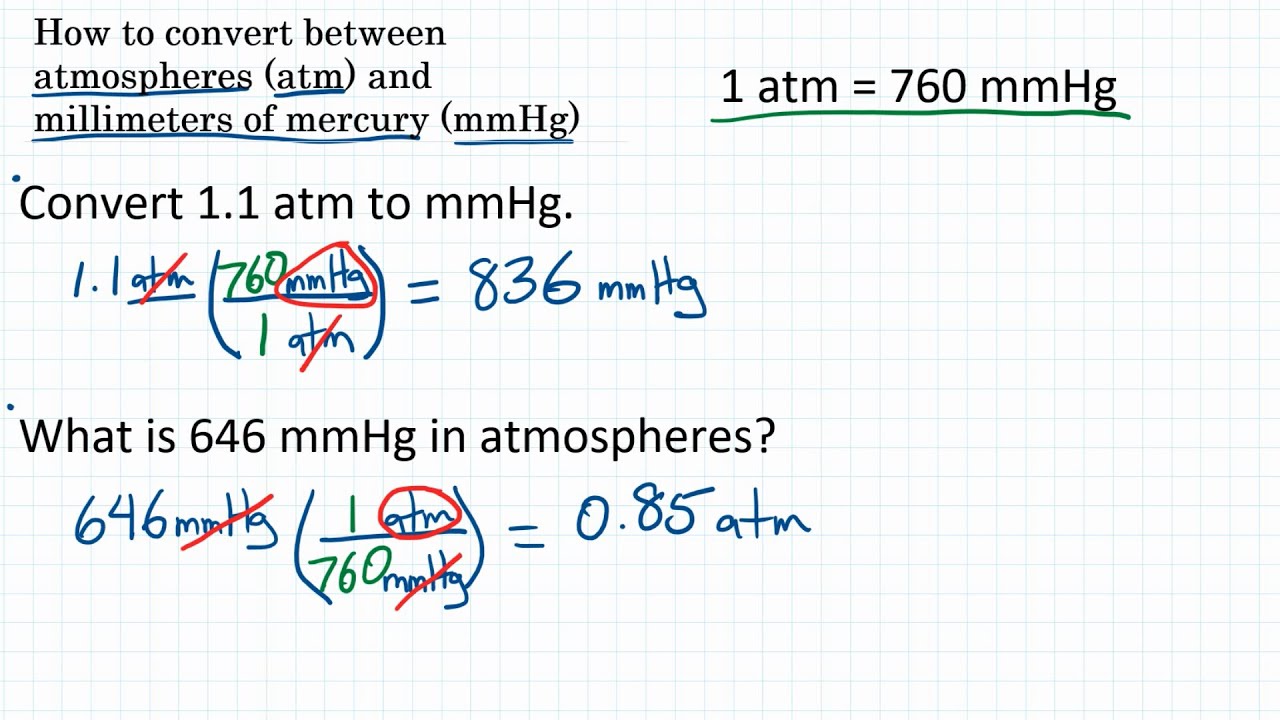
Symbols, abbreviations, or full names for units of length,Īrea, mass, pressure, and other types. A student reads a barometer in the laboratory and finds the prevailing atmospheric pressure to be 687 mm Hg. How many atm in 1 in Hg The answer is 0.033421060942512. You can find metric conversion tables for SI units, as wellĪs English units, currency, and other data. It is approximately equal to Earth's atmospheric pressure at sea level.Ĭonversion calculator for all types of measurement units. It is sometimes used as a reference pressure or standard pressure. The pressure p in atmospheres (atm) is equal to the pressure p in millimeter mercury (0°c) (mmHg) times 0.
#721 mmhg to atm how to#
How to convert Millimeter Mercury (0☌) to Atmospheres 1 millimeter mercury (mmHg) is equal to 0.00132 atmospheres (atm). The standard atmosphere (symbol: atm) is a unit of pressure defined as 101325 Pa (1.01325 bar). 721.9 Millimeter Mercury (0☌) 0.94987 Atmospheres. The unit is named after Evangelista Torricelli, Italian physicist and mathematician, for his discovery of the principle of the barometer in 1643. It is the atmospheric pressure that supports a column of mercury 1 millimetre high. The torr (symbol: Torr) or millimetre of mercury (mmHg) is a non-SI unit of pressure. Please visit pressure conversion to convert all pressure units.You can do the reverse unit conversion fromĪtm to mmHg, or enter any two units below: Enter two units to convert From: To convert torr to atm, multiply the torr value by 0.00131578947 or divide by 760.Ītm (atmospheric pressure) is the force per unit area by the weight of air above that point. 0.94961 Atmospheres (atm) Visit 721.70 Atmospheres to mmHg Conversion Millimeter Mercury (0C) : Millimeter of mercury is a small pressure unit which represents the pressure pushing down due to gravity of any volume of liquid mercury which is 1mm high. If the pressure of the gas is the eudiometer is 721.7 mmHg, what is the pressure of the water vapor +. A barometer shows the atmospheric pressure is 748.2 mmHg. How many grams of helium are needed to fill a balloon to a volume of 5.0L at 22 degrees celsius and 745 torr arrowforward. Torr = atm * 760 How to convert torr (mmHg) to atm?ġ Torr (mmHg) is equal to 0.00131578947 atmospheric pressure (atm). Gas was collected over water using a eudiometer. Calculate the density of argon gas at 23.0 oC and 784 torr. To convert atm to torr, multiply the atm value by 760. How to convert atm to torr (mmHg)?ġ Atm (atmospheric pressure) is equal to 760 torr (mmHg). atm to mm.


What is its volume at 1.39 atm Hint Boyles Law QUESTION 23 A 120g sample of NaCl is dissolved in 48.0 g H,O. If the final pressure in the tank is 721 mmHg, what must have been the original pressure (in atm) in the cylinder 2 1 2 1 V P P x (721 mmHg V 1 atm x 760 mmHg 1875 L ) x 49. Alternatively, to find out the torr value for the most commonly converted atm value, you may check the atm to torr conversion table.īelow, you will find information of how to convert atm to torr and how to convert torr to atm, including the formulas and example conversions. Transcribed image text: QUESTION 21 If atmospheric pressure in Boulder, Co is 721 mm Hg, What is the pressure in units of atm QUESTION 22 A sample of gas has a volume of 7.34 L and is at 1.12 atm of pressure. A 35.8 L cylinder of Argon gas is connected to and transferred into an evacuated 1875-L tank at constant temperature. To convert atm (atmosphere) to torr (mmHg) and to convert torr (mmHg) to atm, you may use the converter above.


 0 kommentar(er)
0 kommentar(er)
